|
General Description of Copper
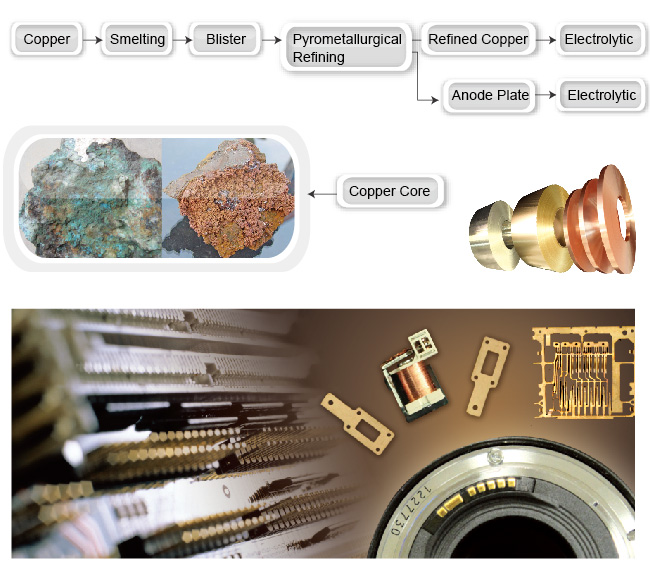
"Copper", chemical element symbol: Cu, specific gravity ( g / cm3 ) = 8.89-8.95, melting point: 1083℃, resistivity: 0.0167Ω mm2/m, linear expansion coefficient: 17.6x10-6/℃, thermal conductivity rate 0-100℃, 399W/mk, flexible status: extensibility rate 280MPA, ≧40%. Copper exists in nature in the form of copper mines. Copper minerals polymerize with other minerals to become copper ores, which are exploited and refined to become refined copper ore with the highest content of copper, then further refined to become copper material.
The Properties of Copper
It has a best feature: Its high electrical conductivity and high thermal conductivity are second only to "silver", ranking the second highest among all metals.
Resistance to corrosion: Copper has a standard electrode potential of +0.345V, which is higher than hydrogen, so in a water solution, it could not be converted to hydrogen. Therefore, copper has excellent chemical stability among all media. In the atmosphere, copper can form on its surface a thin film of CuSO4.3Cu (OH)2 (also known as copper green), which cannot be dissolved with its substrate, serving as a protective layer to prevent further corrosion of the copper core.
Therefore, due to its superior characteristics including electrical conductivity, dependability, machinability and resistance to corrosion, it is often applied to: conductive wire, electric cable, electrical household, multimedia products, as well as industrial chains close to our living circles: 4(C) = Communication, Consumer Electronics, Computer and Cars.
Chemical properties: it has good resistance to corrosion when exposed to Hydrochloric acid or Organic acid (Acetic acid, Citric acid, Fatty acid, Lactic acid, Oxalic acid). But it does not have resistance to corrosion when exposed to non-oxidizing acid (Nitric acid). Strong corrosion will occur when it is exposed to Ammonia, Ammonia chloride, Cyanide, Mercuric salt water solution or moist halogen-group elements.
(At room temperature and in dry air, copper will almost not oxidize. But when the temperature exceeds 100 degrees Celsius, it will expedite oxidization and produce a thin red film of Cu2O.)
Copper Ware
Copper ware: it can be processed to obtain copper rods, copper pipes, copper wires, copper coin (band), and cast into irregular-shaped decorative items.
CDA series |
Determination of ingredients, characteristic actions |
| 1XXXX |
High copper, having a copper content of 95% or more |
| 2XXXX |
Copper zinc alloy (Brass), dependent on its zinc content |
| 3XXXX |
Copper alloy with added lead, used for machining with better cutting Performance |
| 4XXXX |
Special copper alloy, containing at least three (copper, tin, zinc) elements |
| 5XXXX |
Bronze, copper and tin alloy, depending on its tin content |
| 6XXXX |
Copper aluminum alloy, depending on its aluminum content |
| 7XXXX |
Mainly copper nickel alloy, depending on its nickel content |
| 8XXXX |
This series is copper specifically for casting and machining purposes |
| 9XXXX |

|
|